Summary
- Kala Pharmaceuticals got its second ocular therapy, Eysuvis, approved on October 27.
- Peak sales estimates range from $500 million to over $1 billion due to its unique targeting of acute, episodic dry eye that is more common rather than the chronic version.
- Despite this progress, Kala stock has tanked of late, creating a huge opportunity to scoop up shares of this quality company for a discount.
- This idea was discussed in more depth with members of my private investing community, Biotech Value Investing. Get started today »
Kala Pharmaceuticals (KALA) is a platform technology company with already marketed products. Its most important product, Eysuvis, was just approved last month with a launch to follow in December, and expectations are high, with $500 million in peak sales being the lowest estimate I’ve seen. In this article, I review Kala’s proprietary technology, assess Eysuvis in light of both current and future competitors, and discuss the company's valuation and potential upside.
Kala’s Technology Platform has Already Resulted in Two Marketed Products
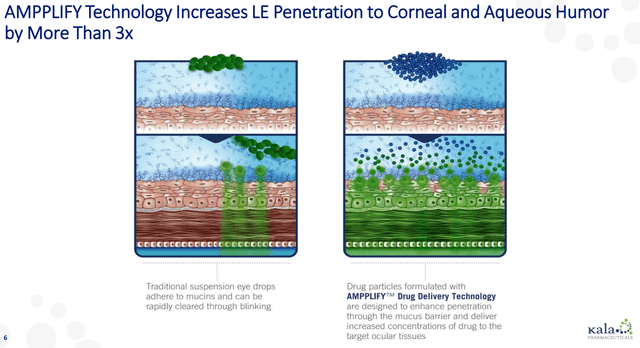
Kala’s products rely on its AMPPLIFY technology platform, which stems from research that originated at Johns Hopkins. AMPPLIFY is a nanoparticle delivery system that allows a drug to not be bound up by mucus and then eliminated by the mucus membranes. This mucus binding ordinarily would allow the eye to eliminate things like dirt, but it can also make it difficult for drugs to penetrate the ocular surface.
Figure 1: Kala’s AMPPLIFY Technology
(Source: Kala’s October 2020 Investor Presentation)
This nanoparticle delivery system not only allows the particles to get into the pores on the ocular surface, but it also makes them neutral from an electrostatic standpoint, which again lets the drug particles get through at higher rates. Increased amounts of the drug then actually make it to the back of the eye, where it can have its effect.
Figure 2: Kala Key Highlights
(Source: Kala’s October 2020 Investor Presentation)
Kala has applied this technology so far in two areas: Eysuvis and Inveltys. From what I’ve heard management discuss, the company originally looked at other areas like pulmonology and oncology, but the sole focus at present and for the foreseeable future is ophthalmology.
Eysuvis is a treatment for acute, short-term dry eye symptoms, and it received FDA approval in October 2020. Eysuvis is a corticosteroid eye drop where the patient typically takes one to two drops up to four times per day. Dry eye disease is caused by ocular surface inflammation, and steroids are well-known to effectively quell inflammation, both in the eye and throughout the body.