Summary
- Biogen had a dramatic day, after years of flip-flop, as it appears the company's new Aducanumab drug will be approved.
- Even outside of Aducanumab, the company has continued to perform well, with an efficient balance sheet and impressive portfolio.
- Long-term, Biogen is an undervalued company with the ability to generate valuable long-term shareholder returns.
- I do much more than just articles at The Energy Forum: Members get access to model portfolios, regular updates, a chat room, and more. Get started today »
Worldwide, 44 million people have Alzheimer's. In the U.S., an estimated 5.5 million people have it, including more than 5 million above 65. That's more than 10% of those above 65. With the world's aging position expected to increase significantly, this proportion will increase significantly. Alzheimer's currently has no FDA approved treatments.
That's why, based on Biogen's (NASDAQ: BIIB) announcement of likely approval of its new Alzheimer's drug, the company's share price increased by 44%, a $16 billion market capitalization increase.
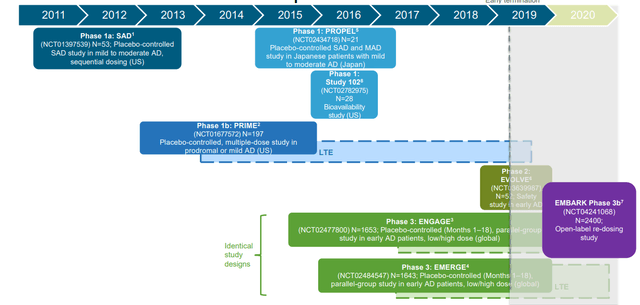
Aducanumab Results Update
The FDA and Biogen have announced a number of recent updates on Aducanumab.
Aducanumab Clinical Development - Aducanumab Investor Presentation
The company has had a volatile share price for several years on the back of volatile news on its Aducanumab drugs. This drug is focused on targeting a plaque that forms in the brain cells of Alzheimer's patients, one of the first drugs to target it, and it has been in development for nearly a decade. Across this time it's gone through numerous studies.
However, recent FDA and Biogen results indicate that re-examining the data could lead to drug approval.
Aducanumab targets the plaque that forms in brain cells of Alzheimer’s patients. After initially concluding that its drug had failed its Phase 3 trial, Biogen re-examined the data and found what it says is evidence that the treatment works.
In its briefing documents, the FDA staff agreed. It said the reanalyzed Phase 3 trial “provides primary evidence of effectiveness,” which is further supported by chemical changes detected in patients, as well as evidence from an earlier Phase 1 study.
In a note following Wednesday’s FDA filings, Wells Fargo Securities analyst Jim Birchenough said he expects Friday’s advisory committee to recommend approval. “While predicting any FDA panel outcome is difficult,” he wrote, “we believe that risk-reward is favorable and view positive recommendation as more likely than a negative vote.”
The initial indication is that the data wasn't sufficient. However, Alzheimer's patients don't have much for them. This drug could be a blessing. Initial indications were just a 20% chance of approval, but the potential for $12 billion peak sales. Biogen is at a low double digit P/E ratio, and this drug could allow the company to double profit.