Summary
- Cara's stock has risen over 100% YTD and 30% over the Last Month.
- Cara has a strong pipeline, making it an attractive long term investment at lower levels.
- If the stock does not pull back in coming weeks, be ready to pounce given the strong catalysts for the stock in 2H of 2019.
Introduction
Cara Therapeutics is a small-cap biotech stock specializing in treating both acute and chronic pain management. In addition, the company is also focused on treating individuals with pruritus, a side effect of many diseases like chronic kidney disease, which causes individuals to develop painful itches.
20 million individuals with pruritus are treated with prescription drugs every year. Thankfully for these individuals, Cara is working to bring several drugs to market to treat their condition. Cara’s main drug KORSUVA is currently in phase II and III trials for oral and intravenous versions. Thus, the company is oriented around bringing this drug to market to show the drugs efficacy in treating various diseases.
What Makes Cara’s Treatment Revolutionary - KORAs
Current methods to treat pain through opiates were successful in reducing patient’s reported pain, but created their own negative side effects, mitigating their overall effectiveness. Cara has stressed that there has been little innovation in the treatment of pain by citing that the date of creation for morphine and acetaminophen was well before the 21st Century.
Cara’s treatment breakthrough stems from its decision to target the kappa opioid receptor, which is located on the peripheral nervous system. Drugs like morphine target the central nervous system (CNS), which lead them to affect the individual’s brain activity. Because Cara’s KORSUVA acts on the periphery rather than the brain, the negative side effects of opioids are mitigated, while reducing an individual's pain.
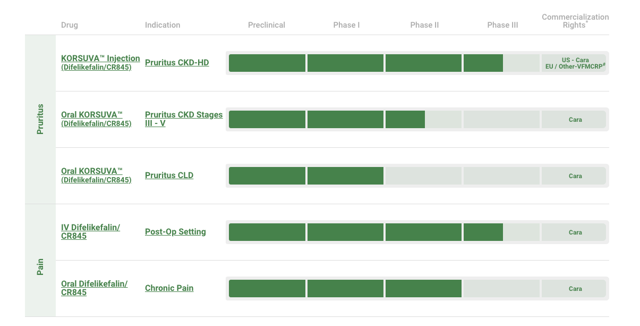
Pipeline
KORSUVA Injection Form
On May 29th, the company reported positive results for its first round of stage 3 trials. The company is currently testing its drug on patients with end stage renal disease, who also commonly develop pruritus during dialysis treatment. In the company’s July investor presentation, Cara stated that a second trial had begun and results would be announced for this trial in the second half of 2019. This second trial will be a pivotal moment for the company, who could be significantly hindered if this drug proves ineffective during stage 3 trials.