Summary
- Biogen's multiple sclerosis revenues are declining, and likely to see continued revenue declines.
- Biogen's growth is heavily dependent upon spinal muscular atrophy medication Spinraza. If Novartis' Zolgensma is approved it will compete with Spinraza, possibly ending Spinraza's growth.
- Since the termination of the Alzheimer's medication aducanumab, share price has been supported by Biogen's share repurchases. Once authorized repurchase money is depleted, share price could fall.
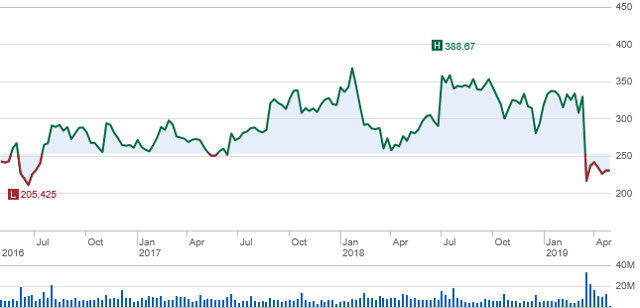
Since Biogen's (BIIB) 2016 strategic shift from multiple sclerosis (MS), two events have caused share price to rise and fall during that period. Those two events were FDA approval of Spinraza for the treatment of spinal muscular atrophy (NYSE:SMA) and the termination of aducanumab for the treatment of Alzheimer's disease (AD).
Source: schwab.com
Without success of aducanumab and declining revenues from the Company's MS product line, earnings are heavily dependent on growth of Spinraza. However, Spinraza growth may be limited because of the pending approval of Novartis' (NVS) Zolgensma for the treatment of SMA expected during May. Zolgensma's approval will likely have a short-term and possibly a long-term effect.
If you are invested in Biogen, consider reducing, or exiting your position. An alternative strategy would be selling a covered call to reduce your cost. If you are contemplating investing, wait until after the FDA decision; if approved shares will likely head lower.